IgG is the most abundant antibody in normal human serum and represents 70-85% of the total pool of immunoglobulins (1). It is monomeric with a molecular weight of approximately 150 kDa, is the main antibody of the secondary immune response, and has the longest half-life (20-24 days) of the five classes of immunoglobulins. IgG consists of four human subclasses (IgG1, IgG2, IgG3, and IgG4), each of which contains a different heavy chain. They are very homologous and differ mainly in the hinge region and in the extent to which they activate the host's immune system. IgG1 and IgG4 contain two interchain disulfide bonds in the hinge region, IgG2 has 4 and IgG3 has 11. Details of functional differences between human IgG subclasses will be described in the section on effector function of Fc. In mice, the IgG class is divided into five subclasses (IgG1, IgG2A, IgG2B, IgG2C and IgG3) and in rats there are four (IgG1, IgG2A, IgG2B, IgG2C). The nomenclature of subclasses has emerged independently for each species, so there is no general relationship between the subclasses of each species.
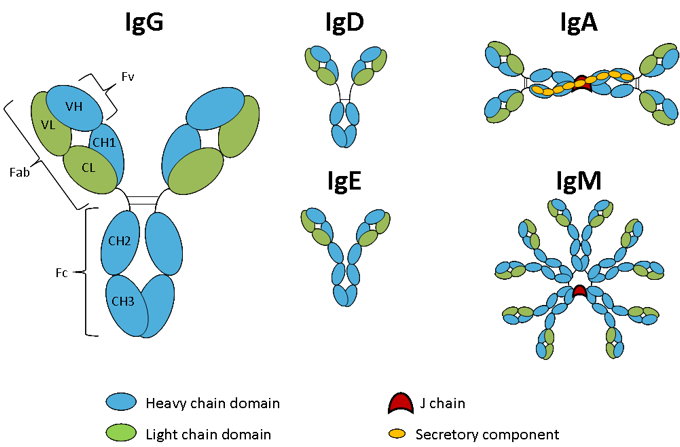
IgM represents 5-10% of the immunoglobulin pool and is the predominant antibody in the primary immune response (1). Unlike IgG, IgM does not contain a hinge region, but contains an additional constant domain and an 18 amino acid tail at the carboxy terminus, which contains a cysteine and participates in the multimerization of the molecule. It is classically represented as a pentamer of the basic four-chain structure that is held together by a J chain, but it can also exist in the hexameric form without the J chain (2) and as a monomer on the surface of B cells. IgM soluble is greater than 1 MDa and, therefore, due to its size, is largely limited to the intravascular pool.
IgA represents approximately 5-15% of the antibody pool and exists as a monomer or dimer (1). IgA is the predominant antibody in mucous secretions such as saliva, tears, milk, and intestinal juices. Like IgM, the multimeric form of IgA contains a tail at the carboxy terminus and is held together by the J chain. The secretory component is a 75 kDa polypeptide chain synthesized by gut epithelial cells for binding to the dimeric form of IgA. As its name implies, the secretory component facilitates the transport of IgA through epithelial cells, but it also participates in the protection of IgA secreted in the lumen of the intestine from proteolytic digestion.
IgD represents less than 1% of total plasma immunoglobulin, but is present in large quantities in the membrane of B cells (1). IgD has the same basic structure as IgG but with an extended hinge region that is highly susceptible to proteolytic digestion. The precise function of this class of antibodies is still unknown.
IgE is very scarce in serum, but it is found in basophils and mast cells of all individuals (1). Similar to IgM, IgE has two additional constant domains in place of the hinge region. This class may play a role in immunity to parasites, but is most commonly associated with immediate type I hypersensitivity, where an IgE immune response occurs to harmless environmental antigens such as pollen and peanuts.