Q1.What is Fluorescence? Is it the same as Phosphorescence?
When an atom or molecule absorbs a photon of light or is otherwise supplied with a sufficient quantum of energy (e.g. by interaction with energetic electrons or as a result of chemical reaction) it reaches an electronically-excited state. For simplicity we will confine the discussion to molecules excited by absorption of light. The state first formed on excitation with a photon of light is almost always a so-called 'excited singlet'
The term 'singlet' means that the net spin of the electrons involved in the excited state transition is zero. This excited singlet state can subsequently give rise to other excited states, particularly the so-called 'triplet' state. Excitation of molecules by means other than photon absorption can lead to direct formation of such 'triplet' states.
An electron can have a spin of +1/2 or -1/2. In the ground state of most molecules electrons are paired and must have opposite spins if they occupy the same orbital. The net spin of the ground state is therefore zero and hence is a singlet. In an excited state the electrons can occupy different orbitals and might have the same spin or opposite spins. An excited state where the net spin is 1 (i.e. both electrons have the same spin) is called a triplet state. Quantum mechanics predicts that transitions from a singlet ground state to a singlet excited state are allowed but those from a singlet ground state to a triplet excited state are forbidden.
The terms 'allowed' and 'forbidden' are not absolute and 'forbidden' transitions can occur, though usually with much lower probability than 'allowed' transitions'. Perturbations introduced by the proximity of heavy atoms and paramagnetic species can have substantial effects on the rates of 'forbidden' transitions and are said to 'relax' selection rules. Once formed an excited singlet state will immediately lose excess vibrational energy and will then interact with its surrounding. First the arrangement of solvent molecules around the excited molecule will change to take account of the different polarity of the excited species. This rearrangement gives rise to an equilibrium excited singlet state of lower energy than that initially formed. An excited singlet state can lose its excess energy in a number of ways. Common examples are:
- interaction with the surroundings leading to energy loss by excitation of vibrations/rotations in surrounding molecules
- emission of a photon of light. THIS IS FLUORESCENCE
- collisional interaction with 'quenching species' such as oxygen leading to deactivation.
- conversion to a lower-energy excited triplet state by so-called INTERSYSTEM CROSSING
- radiationless transfer of energy to a nearby molecule leading to excitation of the neighbour
- the excited molecule might undergo some chemical reaction with surroundings or might break up spontaneously leading to loss of fluorescent species ('PHOTOBLEACHING')
When an excited molecule has converted to an excited triplet, this too can be 'quenched' by interaction with molecules in solution etc. Oxygen is a very effective quenching species, deactivating excited triplets and itself being excited to so-called SINGLET OXYGEN. Singlet oxygen is a short-lived, highly reactive species. Once formed it will react with almost any organic molecule in its immediate surroundings. Thus, singlet oxygen might react chemically with the molecule that led to its formation. Reaction of fluorescent species with singlet oxygen usually leads to decomposition, and hence is a common cause of PHOTOBLEACHING.
The fluorescence process is normally highly efficient. The emission process usually follows a single exponential decay in simple cases ( as does the decay profile of a sample of a radioactive isotope). Typically, the concentration of excited species will decay on a timescale of nanoseconds. The decay time or LIFETIME of fluorescence is defined as the time for the excited state concentration to decay to 1/e (about 37%) of the initial value. This definition is used (rather than the half-life as is conventional for radioactive decay) because it simplifies the relevant rate equations describing the decay mathematically.
An excited triplet can also lose energy by non-radiative means or alternatively it can emit a photon of light. This emission is called PHOSPHORESCENCE. Because the transition involved is 'forbidden' this is a slow process. Thus, phosphorescence is usually emitted over periods of microseconds to seconds compared to nanoseconds for fluorescence. The low rate of photon emission leaves the excited state open to deactivation by other processes that compete with phosphorescence. Thus, phosphorescence is normally a very inefficient process in solution and is usually only important for molecules that have some sort of protection against quenching processes. Solid samples often show phosphorescence, and molecules in solution in 'cages' (e.g. entrapped in cyclodextrins etc) can also phosphoresce.
Conventional phosphorescence measurements are often made in vacuum-degassed frozen glasses prepared from organic solvents. Room temperature phosphorescence is conveniently studied in glasses prepared by drying solutions of sugars and saccharides. Deoxygenation of samples enhances phosphorescence markedly. Heavy atoms such as iodine can enhance phosphorescence by relaxing selection rules for emission and by increasing efficiency of intersystem crossing. Thus halogenated organic molecules are often phosphorescent. An example is the dye eosin, which is a halogenated relative of fluorescein. Fluorescein shows virtually no phosphorescence is solution, whereas eosin shows both fluorescence and phosphorescence.
The terms 'fluorescence' and 'phosphorescence' are often used rather loosely by non-specialists. 'Phosphorescence' tends to be used to describe any long-lived emission, for example the glow emitted by phosphorus as it oxidises in air (this is actually chemiluminescence). Some long-lived emission is actually fluorescence that is emitted as a result of re-excitation of triplet states back to the excited singlet, and technically should be called 'delayed fluorescence'. If the origin of emission is not known, it is best to describe it by the general term 'luminescence'.
[Back to FAQ]
Q2.Why is Fluorescence usually emitted at longer wavelength than the exciting light?
Most molecules in the ground state are in the lowest vibrational energy level. A photon can be absorbed if it provides at least enough energy to raise the ground state to the lowest vibrational level of the excited state. A slightly more energetic photon might excite the molecule to a vibrationally excited level of the excited state, but this will lose excess vibrational energy in picoseconds. Photons of still higher energy might raise the molecule to higher electronically excited states, but in almost all cases these upper states either cause decomposition or very rapidly lose energy giving rise to the usual excited singlet state.
If a molecule is in an isolated environment, such as in a gas phase, then the fluorescence is emitted from the lowest vibrational level of the excited state to the ground state and its vibrationally excited levels. This emission has a spectrum which is a mirror image of the absorption spectrum. Where emission is excited by a narrow spectral souce such as a laser, some emission is at the same wavelength as the excitation, and is known as resonance fluorescence.
For molecules in solution the excited state can usually reduce its energy through rearrangement of the solvent 'cage' around the molecule prior to emission. In this case, although the emission spectrum is often still rather similar to a mirror image of the excitation spectrum, the absorption peak and the emission peak do not coincide. The emission maximum is now at longer wavelength (lower energy) than the excitation. It is said to be RED SHIFTED and the difference in energy between the excitation and emission maximum is called the STOKES SHIFT (Pedantic note: The Stokes shift or Stokes' Shift but, please, not Stoke's shift as is often seen! No need to insult Stokes).
Several factors influence the magnitude of the Stokes shift. If the environment is rigid so that little rearrangement is possible then the Stokes shift is expected to be small. The magnitude of the shift depends on factors such as solvent polarity, viscosity and polarisability. It also depends on whether the excited state can undergo any specific interactions such as proton transfer or charge transfer to other molecules or (sometimes) within the same molecule. Where fluorescent materials are used as detectable labels a large Stokes shift is often highly desirable because it makes life easier when optical filters are used to separate exciting light and fluorecence emission.
Fluorescent labels with very large Stokes' shifts include lanthanide complexes of europium and terbium that are excited in the near ultraviolet region and emit in the orange/red and green spectral regions respectively. These molecules have large shifts because their excitation processes are mediated by energy relay mechanisms from triplet states of ligands bound to the lanthanide ions. Ruthenium complexes and related platinum-metal complexes also have large Stokes' shifts because their emission involves a metal-to-ligand charge transfer in the excited state. Molecules where intramolecular proton transfer takes place in the excited state also show large shifts.
[Back to FAQ]
Q3.Can fluorescence ever be emitted at shorter wavelength than the exciting light?
The simple answer to this is 'yes'. Such emission is known as 'Anti-Stokes' fluorescence and is most commonly seen when absorption and emission spectra overlap substantially. The process involves coupling of vibrational energy from the sample to the electronic excitation, and is rather similar to the effects that give rise to Anti-Stokes Raman spectra. Anti-Stokes fluorescence is not much used for practical applications however, mainly on account of relatively low efficiency.
[Back to FAQ]
Q4.What sort of processes 'quench' fluorescence?
Fluorescence can be quenched by a variety of mechanisms. Common effects are:
- 'Dynamic' quenching where a collisional encounter between the quencher and the excited state is involved. The lifetime and intensity of the emission are decreased by dynamic quenching. So-called 'Stern-Volmer' kinetics apply to the simplest dynamic quenching processes according to the well-known equation (Io/I)-1=K[Q] where Io and I are the fluorescence intensities in the presence and absence of quencher respectively and [Q] is the concentration of quenching species. The proportionality constant K is known as the Stern-Volmer coefficient. Fluorescence lifetimes can be substituted for intensities in the Stern-Volmer equation.
- 'Concentration quenching' where a molecule quenches its own fluorescence at high concentration. The mechanism can, for example, be through radiationless transfer of energy between identical molecules (particularly where the Stokes Shift is small) or through formation of aggregates (common for large molecules such a porphyrins) or via a Stern-Volmer mechanism in solution. Common fluorescent dyes such as fluorescein and its derivatives show marked concentration quenching, both in solution and when used to label macromolecules. Increasing the number of labels bound to a given macromolecule frequently does not give rise to a proportionate increase in fluorescence, and this is troublesome for practical applications.
- 'Static' quenching where an interaction between the fluorophore and quencher is involved. Static quenching can result from the formation of a ground state complex that is non-fluorescent or weakly fluorescent for example. Pure static quenching reduces the intensity of fluorescence but does not necessarily decrease the measured lifetime of emission. Static and dynamic quenching sometimes occur in the same sample, and a full analysis of the lifetime and intensity variations as a function of he concentration of quenching species is required to characterise the system.
- 'Colour-quenching' is a mechanism where photons that are emitted are reabsorbed by a strongly coloured component of the sample. Colour-quenching does not shorten excited state lifetimes.
However, colour quenching is often accompanied by another quenching process based on RESONANCE ENERGY TRANSFER ('FRET'). This is a radiationless process where excited species transfer excitation energy to a neighbour having an absorption that overlaps the fluorophore's emission spectrum. FRET is only efficient for molecules in very close proximity (typically within <10nm) so is only seen in concentrated solutions in absence of specific interactions. Colour quenching can be minimised by reducing the sample pathlength for a given concentration of material. FRET-based concentration quenching can be reduced by dilution.
[Back to FAQ]
Q5.How is Fluorescence Lifetime Measured?
Fluorescence lifetime can be measured in many ways, but three methods are common:
- The sample can be excited by a brief light pulse and fluorescence measurements can be made in real-time after the pulse. If necessary the light source can be repetitive and signal averaging can be used to increase sensitivity
- The sample can be excited with a repetitive weak pulsed light source and the emission can be measured by single-photon counting to construct a statistical histogram of the probability of detecting a photon as a function of time (so-called TIME-CORRELATED SINGLE-PHOTON COUNTING)
- The sample can be excited by a light source that is modulated in intensity at an appropriate frequency and the modulation and/or phase-shift of the emission can be measured. Fluorescence lifetime can easily be calculated from either the phase-shift or from the demodulation of emission relative to excitation. This approach is usually known as PHASE-SHIFT/MODULATION FLUOROMETRY and is said to be a FREQUENCY-DOMAIN measurement.
The term 'fluorometry' rather than 'fluorimetry' is often applied to measurements of fluorescence lifetime-related events. The choice between these methods depends primarily on the available equipment and on the lifetime range of interest. The real-time measurement is easy and cheap if lifetimes are relatively long (e.g. > about 100ns) and a suitable fast pulsed light source is available. Often a low-cost nitrogen laser can be used to provide intense excitation of a sample repeated at low frequency (typically c. 20 Hz). LEDs can also be used for measurements of this type and are a very inexpensive alternative excitation source in appropriate circumstances. Time-correlated single-photon counting is arguably the method of choice for laboratory measurements of short lifetime species but it is expensive and can be relatively slow (though this can be overcome in part by multi-channel detection and use of excitation sources with very high repetition rates).
Frequency-domain measurements can be used to analyse complex decays where time-correlated single-photon counting is commonly used. This requires measurements as a function of modulation frequency of the exciting light source. More commonly, frequency-domain measurements are used for rapid low-cost measurements where the form of the lifetime decay is known in advance (e.g. for sensing applications). The major advantages of this method are speed, accuracy, low-cost and compactness. Ultra-bright LEDs emitting in the near UV, blue and at longer wavelengths are extremely well suited to frequency-domain excitation.
[Back to FAQ]
Q6.What can fluorescence lifetime measurements be used for?
Fluorescence lifetime can be used to detect most common quenching processes, and often allows a correction to be applied to improve quantitation of fluorescent labels. Fluorescence INTENSITY depends on the amount of fluorescent label, on the intensity of the excitation light source and on the presence or absence of quenching processes. Fluorescence LIFETIME on the other hand is normally not dependent on the amount of fluorescent label present (unless some concentration-dependent self-quenching is involved), and does not depend on the intensity of exciting light. Thus, measurement of both lifetime and intensity of fluorescence can often be combined to detect most types of important quenching processes.
In some cases fluorescence lifetime data can be used in conjunction with other techniques to reduce the ambiguity of results. Polarisation imaging is an example of a technique where measurement of fluorescence lifetime variations within a sample can help to improve quantification.
Fluorescence lifetime measurements are particularly useful in fluorescence-based biosensors, where the environmental sensitivity of fluorescence is used to detect an analyte. A good example is the use of a sensor to measure oxygen levels, based on the ability of oxygen to quench fluorescence. A sensor having a fluorescent coating might be degraded by photobleaching over time, or the fluorescent sensing layer might be partially worn away. Coloured contaminants in the sample might reabsorb some of the fluorescent emission from the sensor, and the light source used to excitre fluorescence might not be stable over time. None of these problems affect fluorescence lifetime measurements. A sensor based on lifetime measurement will function even if most of the sensor layer is degraded, because the sensing process is substantially independent of the amount of fluorophore or the level of excitation.
[Back to FAQ]
Q7.What is the difference between fluorescence lifetime imaging and time-gated imaging?
If a sample has both long-lived and short-lived fluorescence (or phosphorescence etc.) it is often desirable to distinguish between these. For example, a long-lived fluorescent label can be detected with high sensitivity against a highly fluorescent background if the latter has a short lifetime. One simple way to accomplish this is to use a 'gated' detector synchronised to the excitation source. If the detector is insensitive during and immediately after excitation but is turned on rapidly thereafter, then only long-lived emission is measured. If a similar measurement is made without 'gating', all emission is seen. The difference between the measurements with and without 'gating' gives primarily the short-lived component of emission. If the detector is a camera of some type, then a time-gated image can be measured.
A fluorescence lifetime image is one where contrast is directly proportional to some function of fluorescence decay time. For example, the image contrast might represent the average fluorescence lifetime measured for each pixel of the image within the selected spectral range. Such an image can be derived from a sequence of time-gated images by calculation, or can be measured by frequency domain methods or time-correlated methods described elsewhere. It is important not to be too pedantic in making distinctions here because these terms are often used loosely. It is usually obvious from the context whether simple background rejection or a more sophisticated measurement is implied. Background rejection based on lifetime differences can be achieved by appropriate manipulation of fluorescence lifetime images as well as by time gating.
[Back to FAQ]
Q8.Is it true that fluorescence is sensitive enough to detect single atoms and molecules?
Yes, but only in particular circumstances which can not always be achieved in a useful manner.
To understand the subtleties a very simple calculation is useful. Consider a fluorophore with the following typical properties: Quantum yield for fluorescence=0.1 (i.e. a photon is emitted once every ten excitations on average. Many labels have higher quantum yields than 0.1) Quantum yield for photobleaching=0.0001 (i.e. on average the molecule decomposes after 10,000 excitations. Many labels are substantially more photostable than this) These figures tell us that a single molecule of our hypothetical label will (on average) emit 1000 photons if it is repeatedly excited until it is bleached irreversibly. The emitted photons must be collected by a lens, optically filtered to reject exciting light and detected by a sensor. Each of these processes introduces significant losses. Combining these reduces the overall detected signal substantially. Overall efficiency is usually in the range 1-5% or more, depending on detector type and collection optics. If the lower figure of 1% detection efficiency is chosen, still 10 detectable photons are recorded from the label. It is routine to detect single photons of light and ten measured counts is easily enough to detect the label with adequate statistical precision.
Note that the figures used are conservative, and considerably more counts can be recorded for single molecules of many labels.
In fact, it is the level of BACKGROUND signal that determines whether we can detect the molecule or not. Using laser excitation focused onto the sample a molecule can be excited to destruction in milliseconds or less, on which timescale most appropriate photodetectors have negligible background noise counts. Thus, the detector need not limit sensitivity. However the matrix in which the molecule is placed also contributes background signals by processes such as Rayleigh and Raman scattering and interfering fluorescence from contaminants.
One way to reduce background is to limit the observed volume. This can be done using confocal optics. The signal is observed over time using a confocal microscope and bursts of photon counts are seen as molecules diffuse in and out of the observed region. Autocorrelation analysis can be used to analyse these fluctuations and to measure the diffusion coefficient of the label. This is a very powerful method to detect single molecules of labelled species, but it is rather difficult to extend the measurement for parallel measurements on many samples.
Another approach to single molecule detection is to isolate molecules at high dilution in inert matrices at very low temperature. Under these circumstances the absorption and emission spectra become very narrow lines. The species of interest can be detected by modulation of the spectra using ultrasonic or magnetic fields and detection of the characteristic fluctuating signals using narrow-band lock-in methods. For many routine fluorescence measurements these approaches are not feasible. Often background signals are very high and this limits detection to hundreds or thousands of molecules at best.
Single molecule detection is not always required, but even so background can be a very serious problem. For routine measurements any method that increases SELECTIVITY of detection will often give improved sensitivity. The lifetime-resolved methods discussed elsewhere offer one means of reducing background and hence of increasing detection sensitivity in routine assay situations. Lifetime resolution can also be combined with other techniques such as chemometric analysis of spectral features to give further improvements.
[Back to FAQ]
Q9.How can an intensified camera give fluorescence lifetime contrast?
There are several types of image intensifier. Early intensifiers ('Gen-I') used a photosensitive cathode and a phosphor screen in a vacuum envelope equipped with focusing electrodes. A very high voltage (several tens of thousands of volts) accelerates emitted photoelectrons which collide with the phosphor screen causing light to be emitted. Intensifiers of this type typically have low 'gain' (i.e. the light from the phosphor screen is not very bright at low incident light level). For this reason such intensifiers were commonly connected in series, so that the screen of one intensifier is optically coupled to the photocathode of another intensifier.
For optimum sensitivity three intensifiers were often connected in this way. The disadvantage of this approach is the considerable bulk of the package and the need to supply very high voltages. Each of the intensifiers has an associated fluctuation or 'noise' in the output and the combination of intensifiers amplifies this 'noise'. It is difficult to 'gate' (i.e. switch on and off very rapidly) an intensifier of this type on account of the high voltages pulses required.
Similar comments apply to attempts to modulate the gain of these intensifiers at very high speed. A further problem is the potential for fast gated intensifiers using very high voltage pulses to radiate electrical noise which can interfere with sensitive electronic equipment. 'Gen-I' intensifiers might be modulated or gated by inclusion of control grids rather like those in a vacuum tube or 'valve', but this is not a common practice.
A second type of intensifier is the so-called 'proximity-focused' or 'Gen-II' intensifier. In this design the photocathode is physically very close to an electron amplifier known as a microchannel plate. A potential of a few hundred volts or so accelerates emitted electrons from the photocathode and directs them into nearby regions of the microchannel plate amplifier. The microchannel plate is composed of an array of tiny channels in a glass ceramic matrix. The walls of the channels are doped so that energetic electrons striking them emit a number of secondary electrons in proportion to the energy of the incident electron. A potential of the order of 800 volts is applied across the microchannel plate. An electron entering the plate gives rise to a burst of secondary electrons from the rear of the plate as a result of a cascade process. These secondary electrons are accelerated by several thousand volts and strike a phosphor screen, giving light output.
Often microchannel plates are stacked in series in intensifiers of this type. One of the advantages of a proximity-focused intensifier is the compact size and the capability of operating at lower voltage than the earlier design.
Proximity-focused intensifiers are easily 'gated' by applying a voltage pulse to the photocathode. For fast gating a pulse of the order of 100 volts or so is adequate. Alternatively the voltage across the microchannel plate can be switched, though this requires a larger voltage change. The gain of a proximity-focused intensifier can also be modulated either by imposing a modulation voltage on the photocathode or by modulating the voltage across the microchannel plate (which is less common and can lead to thermal 'drift' of the intensifier's gain).
If modulated operation at high frequency is required it is usual to operate with a reduced proximity-focusing voltage, so that a relatively small modulation voltage can be used. This helps to keep power dissipation in the intensifier to an acceptable level. If this is not done the photocathode becomes very noisy and can show time-dependent changes in properties. The disadvantage of this mode of operation is that the spatial resolution of the intensifier is degraded because the proximity focus is maintained by the accelerating voltage between the photocathode and microchannel plate.
If fast gating or modulation is required the relatively high resisitivity of the photocathode can give rise to problems. One effect is so-called 'irising' where the response at the centre of the intensifier is delayed relative to that at the periphery and the modulation depth is reduced. This effect is most marked for intensifiers having large photocathode areas. Various attempts to minimise this problem have met varying degrees of success. A partially-transmissive metal undercoat on the photocathode can increase the speed of gated response and improve modulation bandwidth, but at the expense of reduced sensitivity. A fine metallic grid on the photocathode can be used to similar effect, but with potentially less loss of sensitivity.
There are variations on the above themes of intensifier design, based on different types of photocathode and focusing structures. One modern variant uses a semiconductor sensor encapsulated in the vacuum envelope of the intensifier instead of the phosphor screen. This 'electron-bombarded' device gives good results since it avoids the need to form an intermediate image which must be recorded by a camera of some type with inevitable loss of sensitivity, linearity and resolution. These intensifiers are presently much more expensive than the common alternatives however.
All intensifiers are easily damaged by exposure to bright light. Very bright light can adversely affect the photocathode properties if no voltage is applied. Even if no permanent damage results the 'noise' level is much higher for a period after such exposure. If protection circuitry is not built in the intensifier can be ruined if over-exposed while the voltage is applied. Photocathode composition can be tailored to give spectral sensitivity in selected regions. The most usual photocathode types have poor red sensitivity. Extended red sensitivity can be had at the expense of substantially increased 'noise' background.
[Back to FAQ]
Q10.Which is best for Fluorescence Lifetime Imaging? Modulated CCD or Intensifier?
The main advantage of the image intensifier for lifetime-resolved imaging is the ability to switch the sensitivity at very high speed. The modulation bandwidth of a typical 18mm diameter intensifier allows operation at frequencies in excess of 100-200MHz and a typical gating time of the order of 5-10nsecs, depending on the photocathode type and whether a conductive coating is used. Faster operation is possible for specially-constructed intensifiers, and Kentech Ltd quote sub-nanosecond operation for some devices. The main disadvantage of an intensifier from the viewpoint of gated or modulated imaging is the high 'noise' level of the intensified detector.
The noise performance of the intensifier is much inferior to a typical CCD detector and very much worse than a cooled CCD. It is difficult to give a definitive value for the noise performance of an intensifier since this depends very much on how the device is operated and there is considerable variation between intensifiers within a given batch. To minimise noise the intensifier is best operated at the lowest gain that is within the design specification.
The noise level is a crucial factor in fluorescence lifetime imaging using frequency-domain methods with modulated excitation. An intensified camera must typically be used with a integrating framestore to allow many images to be averaged. Alternatively, and preferably, the camera associated with the intensifier can be a slow-scan cooled CCD that allows an image to be integrated in the camera without interference from thermal noise. The latter approach is better in principle than integration of images in the computer because the readout noise is lower and is not repeated with each image that is accumulated.
As a rough guide, images must be measured over integration times of several seconds to several minutes to give reasonable performance for time-resolved imaging with an intensified detector in our experience. In part this is because the operations of fluorescence lifetime imaging involve several image subtractions and ratiometric measurements which amplify noise in images. If fluorescence lifetimes of interest are long enough for the alternative PRS directly-modulated/gated CCD technology to be used, we believe this to be a very much better method than the use of intensified detectors. Our opinion is based on several years of experience with both techniques. For this reason PRS has expended much effort in developing CCD technology and we would only recommend the use of intensified detectors where they offer particular advantages of speed.
[Back to FAQ]
Q11.Which light sources are most suitable for fluorescence lifetime measurement and imaging?
The answer to this question depends on many factors such as the lifetime and spectral excitation ranges of interest, budget, required stability levels, size and weight considerations etc. A brief and necessarily incomplete summary is as follows:
Xenon flashlamps
For long lifetime samples (>20 microseconds or so) a fast pulsed Xenon flashlamp is a good compact light source. This gives output from UV to IR and is intense, compact and relatively inexpensive. Some flashlamps suffer from significant 'afterglow', particularly at longer wavelengths and this aspects needs to be investigated for each application. Xenon flashlamps are typically pulsed at several hundred Hz with microsecond pulse widths. Faster flashlamps with sub-microsecond pulse widths are available commercially for special applications. Nanosecond pulsed Xenon flashlamps have also been described in the literature (see Iwata, I. et al. Rev. Sci. Instrumen. 71(11), 4045-49, (2000)).
Nitrogen/Dye lasers
Nitrogen lasers and dye lasers are also very suitable for time-resolved measurements. Typical pulse widths are between 300ps and 10ns and repetition rates are usually up to c. 20Hz for low cost commercial systems. Basic Nitrogen lasers are compact and reliable and give very high peak power levels (hundreds of kilowatts or more) at average power levels of a few milliwatts for a small laser. Cost is typically a few thousand dollars for a sealed unit that requires no external nitrogen source. The output is at 337nm in the UV, which can excite most fluorescent materials, but gives a lot of background from optical filters, lenses etc. Addition of a dye module gives access to a wide range of wavelengths across the visible spectrum.
Ultrabright LEDs
Ultrabright LEDs are becoming very popular. These are available in a variety of colours, but we have most experience with the UV (370-390 nm), blue (470nm peak) and green diodes (530nm peak). A 'white' LED is also available, where a phosphor coating is used for wavelength conversion. Some of these diodes can be pulsed in nanoseconds or less and modulated at frequencies up to c.1-200MHz. Care must be exercised as some diodes have substantial batch-to-batch variations and not all 'colours' are equally suitable for very high speed use. The minimum pulse width of the phosphor-coated white diode is of the order of 100ns in our experience based on very few samples. This relatively slow response is probably limited by the phosphor coating. The LEDs are extremely stable in operation and offer high intrinsic brightness because of the small source size. Output power levels of the order of 1mW are typical but higher power can be obtained with special precautions.
PRS have considerable experience of the use of such diodes for excitation of fluorescence and in our opinion this source is the first choice for pulsed and modulated operation if the emission wavelength is suitable for the label of interest.
Deuterium Lamps
Deuterium lamps were used in much of our early work on fluorescence lifetime imaging. The deuterium source is compact and emits across a wide range of wavelengths from far UV to IR. We have modulated specially-made deuterium lamps (produced in collaboration with Cathodeon Ltd) at frequencies up to more than 100MHz using RF power drivers. The source is very stable with low ripple in both long and short term. The main disadvantage of the deuterium source is the relatively low intrinsic brightness which makes it difficult to use for high-sensitivity fluorescence microscopy. The source aperture is small, but the emission comes from a significant depth within the discharge region, which limits attainable power density at the sample when a high NA objective is used to collect light.
Externally modulated Light Sources
In theory any light source can be used for fluorescence lifetime imaging if it used with an external device such as a chopper wheel, Pockels cell, or acousto-optic modulator. In practice Pockels cells and acousto-optic modulators are best used with highly collimated light sources such as CW Argon Ion lasers and CW frequency-doubled diode-pumped YAG lasers. Whilst we have previously shown that the output from a mercury lamp can be successfully modulated at radio frequencies with a large aperture Pockels cell, the optical losses associated with collimation and the intrinsic instability of these lamps make them less than ideal for lifetime imaging.
In addition to the above light sources a variety of intrinsically modulated lasers are available and many of these have been used for fluorescence lifetime imaging. It is worth mentioning that laser sources can give problems in conventional imaging geometries because of speckle patterns and interference patterns caused by multiple reflections from optical surfaces. Fluorescence lifetime imagers based on laser scanning offer an alternative approach that is particularly suitable for confocal measurements, for example in biological microscopy.
[Back to FAQ]
Q12.How many 'bits' resolution are needed for fluorescence lifetime imaging?
This is a deceptively simple question but the answer needs some careful thought. The first thing to remember is that the noise performance of the 'weakest link' in the imaging chain is the determining factor in the performance of a lifetime imaging system. If the 'front-end' of the imaging system is an image intensifier then this is almost certainly the noisiest component. Even if the camera viewing the image intensifier's screen only worked to 5-bits resolution (i.e. 32 grey levels) this would usually be better than the performance of the intensifier.
At first sight this seems to contradict the information in published accounts (including our own papers), where cameras capable of 12-16 bits are used to collect data. In fact, there is no contradiction. The process of measuring a fluorescence lifetime image involves image manipulations such as subtractions and ratiometric measurements. If the resulting image is to be of high quality then the signal-to-noise ratio of the precursor images must be very high since the image processing operations inevitably degrade signal-to-noise. The best way to achieve the required signal-to-noise figure for an image to be used in such a calculation is to integrate the signal over time (i.e. to perform a time-average) until the data are adequate for the required purpose. This can be achieved by averaging many 'noisy' images acquired using a camera with a low-resolution digitiser or alternatively the signal can be accumulated for an extended time period in a camera with a wide dynamic range (such as a cooled CCD camera.
Whether the in-camera integration offers real advantages over in-computer integration depends on the level of readout noise from the camera relative to that of the primary detector (i.e. the image intensifier in the present example).
[Back to FAQ]
Q13.My CCD camera is capable of 14-bit resolution. What does this really mean?
The bit-resolution of a CCD camera relates to the operation of the A/D converter that digitises the charge packets in the camera. A 14-bit converter can resolve the full-scale output of the camera into 16,384 levels. However, this does not mean that the data recorded are valid to this precision. To illustrate this, consider a typical CCD camera with pixels each capable of storing up to about 200,000 charges (electrons or 'holes' depending on the camera design). Statistically the standard deviation of 200,000 randomly collected charges is the square root (about 447 charges). Thus, although we can measure the voltage level corresponding to the full well capacity of the pixel to very high precision with our A/D converter, we do not know the 'true' value of the photon count to anywhere near this precision
It is reasonable to ask why high precision converters offer advantages in CCD cameras and similar devices. The answer is that the converter allows us to make measurements on high and low charge levels within a given image without having to change the 'gain' of the output amplifier to match the charge level to suit the converter for each pixel. Thus, for example, if an eight-bit A/D converter is used to digitise signals from a device with a full well capacity of 200,000 electrons the least number of electrons that can be sensed is 200,000/256 or about 781 charges. The standard deviation of 781 electrons is about 28 electrons. Another well having only 400 electrons with a standard deviation of 20 electrons would not be distinguished from the higher level using an eight bit converter, even though statistically the count levels in the two pixels are clearly distinct. A higher resolution converter avoids this problem.
Another reason to use a high precision converter is that often signal levels in CCD cameras are increased at the expense of spatial resolution by combining the charge from adjacent pixels (pixel 'binning'). This can often be achieved within the CCD chip itself, leading to increased precision in measurement at the readout stage. The charge capacity of the readout stage is sometimes greater than that of an individual pixel to make this possible. A high-precision A/D converter allows the gain of the output amplifier to be set to suit the higher signal levels seen in the 'binned' pixel blocks while still allowing adequate resolution if the camera is used without pixel binning.
[Back to FAQ]
Q14.What is multiphoton excitation? Why is it used for microscopy?
The energy gap between ground state and excited states of most molecules is such that excitation in the visible or UV range is appropriate. Normally such excitation is due to absorption of a single photon of UV or visible light, which is an efficient process. However it is possible to excite a molecule by simultaneous absorption of two or more photons of lower energy, so long as the sum of the photon energies is high enough to populate the excited state. The process is often called frequency 'upconversion', as the emitted fluorescence is more energetic than the photons of exciting light. For upconversion to happen the photons must be absorbed simultaneously, so the process is most efficient for excitation with fast pulsed lasers where all of the energy is concentrated into a very short time. Essentially it is the power density that determines the probability of excitation in these circumstances. Excitation is often said to proceed via a 'virtual' intermediate state, which can be considered to have an extremely short lifetime. Although it is certainly possible to use two lasers emitting at different wavelengths for selective multiphoton excitation by far the most common experimental arrangement uses a multiphoton excitation with a single laser. Best efficiency is obtained using lasers emitting pulses in the sub-picosecond time range (100 femtoseconds or less is common), though much longer pulses have been used for some purposes.
For microscopy the multiphoton excitation mechanism is of interest because it allows selective excitation of molecules only at the focus of the laser where power density is highest. This means that selective photochemistry can be achieved within a tissue sample without significant photodamage to regions above and below the focal plane. Where the aim is to excite fluorescence the multiphoton excitation is effectively self-confocal for this reason. A scanned multiphoton excitation source gives excellent depth sectioning without the need for conventional confocal optics.
Confocal fluorescence microscopy with a multiphoton excitation source is particularly well suited to use with scattering or pigmented samples where conventional confocal fluorescence works poorly. Near IR light (particularly in the range of 700-900nm) is poorly absorbed by most chromophores and also penetrates scattering tissue samples much more efficiently than shorter wavelengths.
Most usually multiphoton excitation is a two-photon process, but higher order processes are also possible and experimentally realisable without undue difficulty. Three-photon excitation for example gives access to infrared excitation of molecules that would normally need a short wavelength UV stimulus.
The selection rules for excitation by multiphoton absorption differ from those for single photon absorption, and limiting values of fluorescence polarisation anisotropy are also different. These differences have been exploited for experimental spectroscopic studies, but are perhaps of less consequence for routine microscopy. However the short excitation pulses are very well suited to time-resolved fluorescence detection and commercial systems using time-correlated photon counting are available.
The main disadvantage of multiphoton excitation is probably the cost. Femtosecond pulsed lasers are presently very expensive though their prices are falling. In addition the multiphoton process is not appropriate to a wide-field excitation as in conventional microscopy, but must normally be used in a scanning mode to maintain adequate power density at the sample.
Some inorganic materials are known that can give upconversion at much lower power density than is needed for typical organic fluorophores. These materials, known as 'upconversion phosphors' are based on lanthanide ions within a matrix that has very low phonon energy (typically heavy metal fluorides, oxides and oxysulphides for example). The lanthanides can be excited by a number of mechanisms but the most efficient involves a real intermediate excited state (as opposed to the 'virtual' state of a typical organic fluorophore). This can be populated by IR absorption (or energy transfer from a sensitising ion) and subsequently re-excited to a higher excited state that can emit upconverted radiation. The key to the efficiency of excitation for these materials is the long lifetime of the intermediate excited state, which allows ample opportunity for further excitation before vibrational deactivation. Upconverting materials based on this approach are the subject of considerable interest for possible labelling applications in biology, but their use is presently limited because nanoparticles are needed for many applications and these are currently under development.
Finally, there is another type of upconverting phosphor, which is much used for detection of infrared laser beams. This is the so-called electron-trapping or 'storage' phosphor. Materials are based on hosts such as strontium and calcium sulphide doped with lanthanide ions such as europium and having samarium as a further dopant. The phosphors are 'charged' by exposure to energetic radiation (visible light, UV, X-rays or emission from a radioactive source) resulting in production of separated electron-hole pairs in the matrix. Excitation with infrared light can liberate electrons from their 'trapped' state resulting in recombination and emission of visible light. Storage phosphors have not yet found significant use as labels in biology because they are often unstable in aqueous media and have not yet been extensively studied as nanoparticles. Phosphors based on doped barium fluoride-bromide and related materials have found extensive use in biology for detection of radioactive isotopes on gels and blots however as replacements for X-ray film and storage phosphor screens are commercially available for this purpose. The screens are exposed to the labelled sample for a period of time and then scanned with IR radiation to excite regions where the radiation has 'charged' the phosphor.
[Back to FAQ]
Q15. What are Quantum Dots?. Why is everybody talking about them?
Fluorescent labels are usually fairly simple organic molecules, but inorganic materials can also be used. The photophysical processes responsible for light emission in inorganic materials do not always fit the definition of 'fluorescence', strictly speaking, and the more general term 'photoluminescence' is more appropriate. This is a slightly pedantic detail and often 'fluorescence' will be used loosely in the literature. The best known inorganic photoluminescent materials are phosphors such as are used in colour TV CRTs and in 'fluorescent' lights. There are a number of different types of phosphor and this is a complex area, but recently one type of phosphor material has become important for biological labelling. This is the so-called 'quantum dot'. Quantum dots are nanoparticles of semiconductor material (usually materials like zinc sulphide, cadmium sulphide, cadmium selenide and cadmium telluride) that have an unusual property. If the materials are prepared in 'bulk' form they show characteristic broad absorption and emission spectra. Bulk materials such as zinc sulphide, for example, have often been used in the green screens of computer monitors, and their properties can be tailored by 'doping' with low levels of other elements such as silver and copper. If however the materials are prepared as nanoparticles (usually in the range up to a few nanometres in diameter) the spectral properties of the undoped materials change and become dependent on the particle size. In particular, the absorption spectrum is typically very broad and the emission spectrum becomes well defined and relatively narrow.
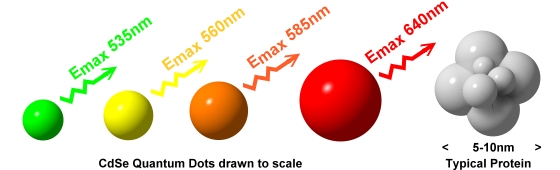 |
Unlike most organic fluorophores where the emission peak is typically followed by a long 'red tail' in the spectrum, quantum dots have a relatively sharp spectral cutoff after their main emission peak. Small particles emit at relatively short wavelengths whereas larger particles emit at increasingly longer wavelengths for a given semiconductor material. Most commonly the size range of the particles is in the 3-7nm region, similar to that of a small protein. If they become much bigger than this then the emission becomes more like that of bulk material. The change in emission properties is known as 'quantum confinement' and is related to the ratio of the particle size to the so-called 'Bohr radius' of the excited species. The latter term is by analogy with the old 'Bohr atom' model where an atom was seen as a central nucleus with an orbiting electron at a fixed radius:- in the semiconductor excitation leads to formation of photo-generated electron-hole pairs and these have a characteristic interaction energy which can be related to a notional 'radius' for the bound electron-hole state.
Quantum dots are of interest for several reasons:
- they have broad absorption so a range of different labels can all be excited by a single light source
- if a single label is used the broad absorption means efficent excitation by broad non-laser sources
- they have high quantum yields for emission and high exctinction coefficients for absorption
- they have sharp emission so it is easy to discriminate spectrally between different labels
- the easy discrimination increases the dynamic range of measurements with many labels
- they are relatively photostable
- using a range of different materials and sizes emission from the violet to the near infrared is possible
- the emission is complex but many labels have major components with long lifetimes (>30ns or more)
- the absence of a 'red tail' in emission makes them very suitable as energy donors for 'FRET' assays
- the long lifetime makes it easy to discriminate QD emission in the presence of conventional labels
- the long lifetime makes them well suited to time-resolved bioassays, (e.g. time-resolved 'FRET')
In order to use the labels in biological applications it is usually necessary to functionalise the surface to allow attachment of proteins, nucleic acids etc. There are a number of ways of doing this ranging from passive adsorption to functionalising the surface with materials such as mercaptoacetic acid and similar molecules to introduce groups that can be linked to other molecules by conventional chemistry.
It is common to grow a 'shell' of another semiconductor on the surface of a quantum dot to improve quantum efficiency. For a given 'core' material such as cadmium telluride, the shell is usually another II-VI semiconductor chosen to have a higher 'bandgap' (e.g. zinc sulphide) to confine the excitons in the core. The 'shell' not only helps to increase quantum yield but it minimises some quenching processes. Quantum dots can be quenched by electron transfer to materials in solution, but are otherwise relatively photostable, though they can photo-oxidise in some circumstances. They do however show an unusual effect in that individual QDs can 'blink' sometimes entering 'dark states' for a period during which they are quenched. The effect is not well understood but has been associated with transient ejection of electrons into metastable surface states. Time-dependent variations of spectral maximum have also been observed in measurements on individual QDs.
At present the main limitation of QDs as labels is perhaps their expense. Because the spectrum is dependent on particle size it is necessary to obtain a monodisperse (uniform-sized) population for best results. Although preparation methods are fairly simple the uniformity of the product is not always easy to control and some form of chromatographic fractionation is often used, increasing production costs. Also, the labels need to be derivatised for biological applications. However, there are commercial pressures which will make these labels more affordable over time. The relatively large size of QDs might also be a problem in some circumstances, for example when rapid diffusion or easy penetration into a matrix are required. This is exacerbated if they are derivatised with relatively bulky proteins such as avidin.
An interesting application area is in imaging through living tissues. The optimal transmission window for living tissue is in the far red (typically between about 650nm and 900nm). The availability of QDs with absorption at long wavelength combined with long wavelength emission minimises effects of tissue autofluorescence and scattering and allows efficient detection using red-sensitive CCDs. The use of time gating further enhances the ability to reject background signals and this is likely to be an important issue in future work.
A possible problem with QDs is the toxic nature of the materials from which they are presently made, though this is minimised by the minute quantities of materials that are typically used and their insolubility. However work is underway to develop QDs from materials such as zinc oxide, which is much less toxic than the typical heavy metal materials.
The emission of typical QDs is well-detected by the Imagex time-gated camera systems and the NanoCCD easily has the time resolution for selective imaging of QDs in the presence of conventional labels. We believe this is an important application area for our technology, both for multiplexed detection in assays and for microscopy and for time-resolved tissue imaging.