During the last two decades, there has been a great interest in RNA-based technologies for the development of prophylactic and therapeutic vaccines. Preclinical and clinical trials have shown that mRNA vaccines provide a safe and durable immune response in animal and human models. In this review, we summarize the progress of current research on mRNA vaccines, which have the potential to be rapidly manufactured and become powerful tools against infectious diseases, and highlight the bright future of their design and applications.
Introduction
Vaccination is the most successful medical approach to disease prevention and control. The successful development and use of vaccines has saved thousands of lives and vast amounts of money. In the future, vaccines have the potential to be used not only against infectious diseases but also for cancer as a prophylactic and treatment tool, and for the elimination of allergens (1-3). Before the 1980s, vaccines were developed for protection against disease-causing microorganisms. Empirically, inactivated vaccines were produced by heat or chemical treatment, and live attenuated vaccines were generally developed in animals, cell lines, or unfavorable growth conditions.
During vaccine development, the mechanisms involved in conferring immunity were unknown. However, the use of live attenuated or killed whole organism vaccines was highly successful in the control and eradication of a number of serious human infectious diseases, including smallpox, polio, measles, mumps, rubella and infectious diseases of animals, such as the classical ones. swine fever, rinderpest and equine infectious anemia. More recently, live attenuated vaccines (LAVs), based on subunits and peptides, have been developed thanks to advances in molecular biology theory and technologies.
The results obtained with LAV vaccination dramatically expanded our understanding of the mechanisms related to the immune response elicited by these vaccines. In the case of inactivated vaccines, antigen-specific antibodies contribute greatly to the prevention and control of infectious diseases initiated by microbes. In addition to specific humoral immune responses. LAVs elicit strong cellular immune responses, which are critical to eradicating many intracellular pathogens.
However, the failures that are sometimes caused by inactivated vaccines are attributed to mutation of the surface antigens of pathogens. Other concerns about LAV applications include the potential to cause disease in immunosuppressed individuals and the possibility of reversion to a virulent form due to back mutation, acquisition of compensatory mutations, or recombination with circulating transmissible wild-type strains (4, 5) . However, subunit and peptide vaccines are less effective in eliciting a robust CD8 + immune response, which is important for intracellular pathogens, including viruses and some bacteria (6, 7).
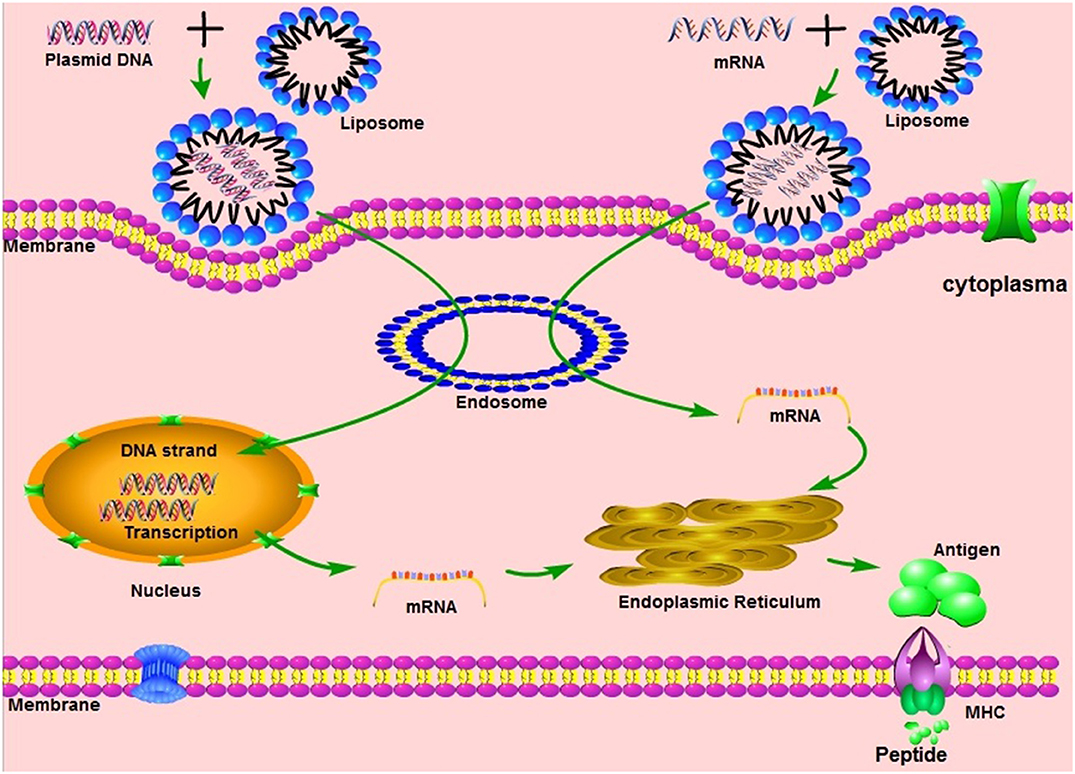
Vaccination with non-virally administered nucleic acid-based vaccines mimics infection or immunization with live microorganisms and stimulates a potent immune response of follicular T helper B cells and germinal center (8, 9). In addition, the manufacture of vaccines based on non-viral nucleic acids is safe and time-saving, without the growth of highly pathogenic organisms on a large scale and with less risk of contamination with live infectious reagents and the release of dangerous pathogens. In particular, for most emerging and re-emerging devastating infectious diseases, the main obstacle is obtaining a reserve on short notice (10). Non-virally delivered nucleic acid-based vaccines can bridge the gap between a disease epidemic and a desperately needed vaccine (10).
Non-viral nucleic acids are classified as DNA or RNA based on their 5-carbon sugar type. From administration to antigen expression, DNA vaccine and RNA vaccines are processed through different pathways. In the steps between immunization with a DNA template and expression of the target antigen, the DNA must pass the cytoplasmic membrane and the nuclear membrane, transcribe into mRNA and return to the cytoplasm and initiate translation (see Figure 1). Although promising and with demonstrated safety, good tolerability and immunogenicity, DNA vaccines were characterized by suboptimal potency in early clinical trials (11). Improved delivery technologies, such as electroporation, have increased the effectiveness of DNA vaccines in