Nissa has a master's degree in chemistry and has taught high school science and college-level chemistry.
When we look at the atomic number of an element on the periodic table, we may not know it, but these elements can have isotopes. This depends on the number of neutrons. In this lesson, we will learn about the three isotopes of hydrogen.
What are isotopes?
Imagine identical twins or identical triplets: they all look the same on the outside, but when we look closer, we notice small physical differences, such as their fingerprints. Also, as we get to know them more individually, we will notice subtle differences in their preferences and personalities.
Let's compare these identical twins and triplets with the isotopes of an element. Like twins and triplets, we can think of isotopes as different versions of an element. Isotopes are different versions of the same element that have the same atomic number but different number of neutrons. Because the number of neutrons is different, they also have different atomic masses, the total number of protons and neutrons combined.
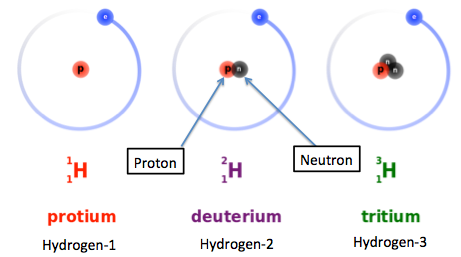
As an example, let's take a look at hydrogen isotopes. Hydrogen has three isotopes: hydrogen-1 (protium), hydrogen-2 (deuterium), and hydrogen-3 (tritium). In the following illustration, we can see subscripts and superscripts. The superscripts 1, 2, and 3 written before H are the atomic masses of hydrogen isotopes, and the subscript 1 is the atomic number. We can see here that the atomic numbers (or number of protons) of hydrogen isotopes are the same, but their neutrons and atomic masses are different.
Three isotopes of hydrogen
Previously, we have shown the three isotopes of hydrogen: protium, deuterium, and tritium. Protium is also known as hydrogen-1, deuterium is also known as hydrogen-2, and tritium is also known as hydrogen-3.
Let's compare how these hydrogen atoms differ in the following table. We can see that for symbols, the superscripts before H are the atomic mass and the subscripts are the number of protons or the atomic number. Protium is also called hydrogen-1. The same is true for the other two isotopes of hydrogen.
Three isotopes of hydrogen
The three isotopes of hydrogen are illustrated here:
Illustration: Hydrogen Isotopes
In addition to the number of neutrons and the atomic number, these hydrogen isotopes also differ in terms of their natural abundance. When we say natural abundance, for isotopes, this refers to the abundance of that isotope found on the planet. The natural abundances of hydrogen isotopes are shown in the following table.
Hydrogen isotopes: natural abundance
Protium (hydrogen-1) has an atomic mass of 1.00782504 and is a stable isotope. It has a proton and it has no neutrons. Protium is also known as ordinary hydrogen. In terms of its natural abundance, it is the most common of all hydrogen isotopes.
Deuterium (hydrogen-2) is the second most abundant isotope of hydrogen, constituting 0.0026 to 0.0184% of the hydrogen found naturally on Earth. Its atomic mass is 2.01410178 and it has one proton and one neutron. Because the nucleus of deuterium is twice as heavy as that of protium, deuterium is also known as "heavy hydrogen." This was discovered in 1931 by an American chemist named Harold C. Urey.