One of the many ways paleoclimatologists learn about past ocean conditions and weather is by using the chemical composition of rock and fossil samples. Remember that chemical elements are made up of a certain number of protons, neutrons, and electrons. The elements have a charged equilibrium (neither positive nor negative) because they have the same number of electrons and protons. However, various chemical reactions in nature will cause elements to gain or lose electrons, and elements to become positively or negatively charged. When this happens, the elements turn into ions.
The positive and negative ions will attract each other to form solids, some liquids, and some gases. When a solid dissolves in water, the positive and negative ions break up and dissociate through the water. Most rocks and hard fossil parts are made of ionic compounds.
For example, table salt, sodium chloride, will dissolve in water to form the positively charged sodium ion and the negatively charged chloride ion. This forms an aqueous (water-based) solution:
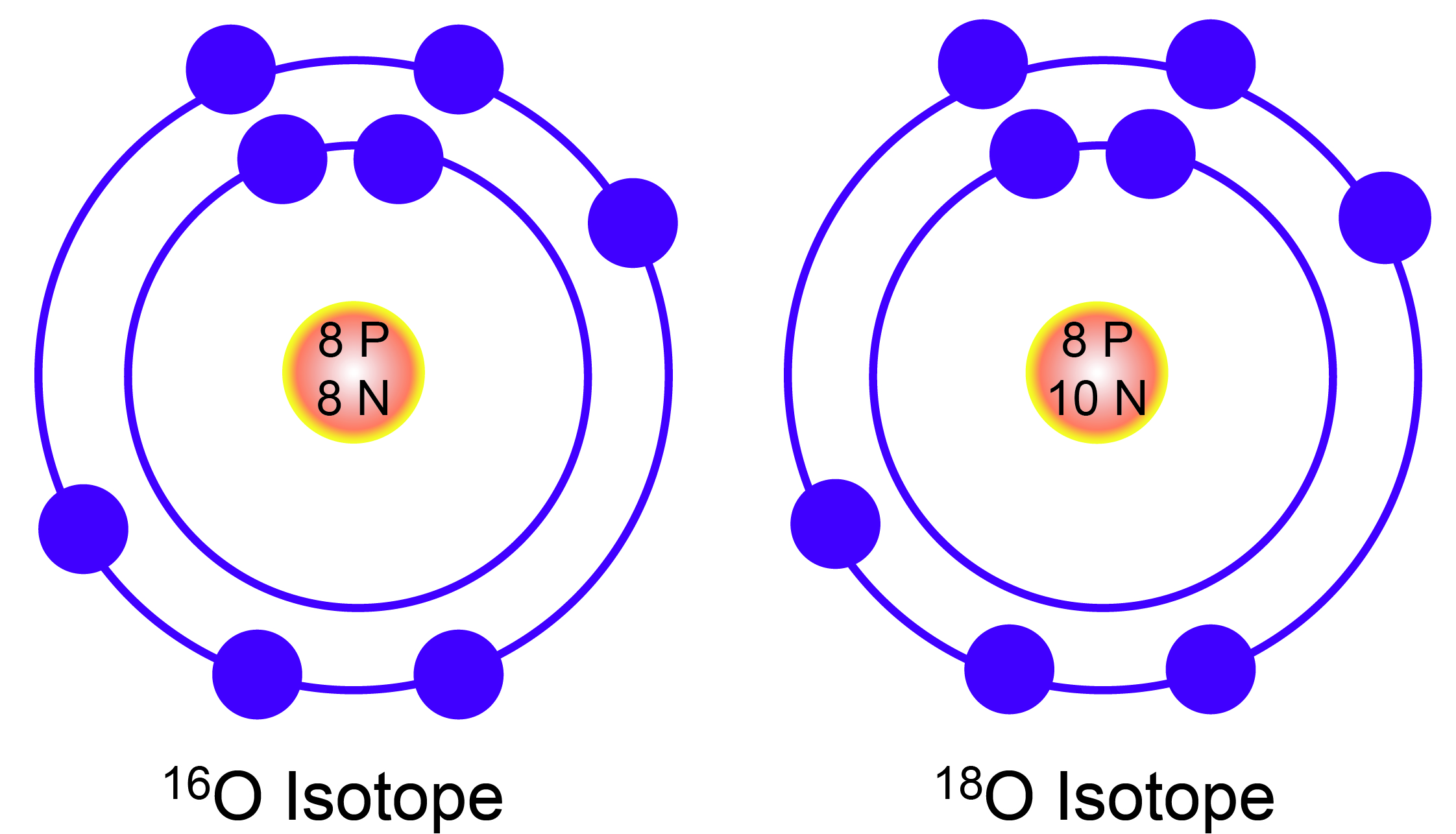
Chemical elements are in different versions, called isotopes. Isotopes are elements that contain the same number of protons, but differ in the number of neutrons in their nuclei. For example, there are three isotopes of the element oxygen (O): Oxygen 16, 17, and 18. Each isotope of oxygen contains 8 protons, but differs in the number of neutrons. An isotope number is a shortened representation of its mass. Because protons and neutrons are roughly equal in mass, the number of an isotope is equal to the sum of its protons and neutrons. Therefore, oxygen 16 has 8 protons and 8 neutrons, oxygen 17 has 8 protons and 9 neutrons, and oxygen 18 has 8 protons and 10 neutrons.
There are two main types of isotopes that geoscientists use to interpret ancient Earth: stable and unstable isotopes. An unstable isotope undergoes radioactive decay, where the element will lose energy over time. Several radioactive isotopes occur naturally and not all of them are bad or cause harm to humans. However, paleoclimatologists do not usually work with these unstable isotopes. Instead, we use stable isotopes that do not undergo radioactive decay.
Two of the most common stable isotopes used by geoscientists are carbon (C) and oxygen (O). Although there are several types of stable isotopes, we will mainly talk about carbon and oxygen obtained from planctic and benthic foraminifera as they are very common in paleoclimatology (especially for studying our oceans), but we will also briefly touch other proxies used for isotopes. analysis.