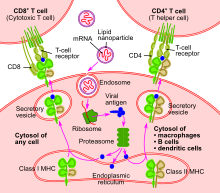
An mRNA vaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. [1] The vaccine delivers antigen-encoding mRNA molecules to immune cells, which use the engineered mRNA as a template to build a foreign protein that would normally be produced by a pathogen (such as a virus) or by a cancer cell. These protein molecules stimulate an adaptive immune response that teaches the body to identify and destroy the corresponding pathogen or cancer cells. [1] The mRNA is administered by a co-formulation of RNA encapsulated in lipid nanoparticles that protect the RNA chains and help their absorption into cells.
File: Coronavirus mRNA vaccines.webm
Video showing how vaccination with an mRNA vaccine works Reactogenicity, the tendency of a vaccine to produce adverse reactions, is similar to that of conventional vaccines without RNA. [4] People susceptible to an autoimmune response may have an adverse reaction to messenger RNA vaccines. [4] Advantages of mRNA vaccines over traditional vaccines are ease of design, speed and lower cost of production, induction of cellular and humoral immunity, and lack of interaction with genomic DNA. [5] [6] While some messenger RNA vaccines, such as the Pfizer-BioNTech COVID-19 vaccine, have the disadvantage of requiring ultra-cold storage prior to distribution, [1] other mRNA vaccines, such as Moderna vaccines, CureVac and Walvax COVID-19 do not have such requirements.
In RNA therapeutics, messenger RNA vaccines have attracted considerable interest as COVID-19 vaccines. [1] In December 2020, Pfizer - BioNTech and Moderna obtained approval for their mRNA-based COVID-19 vaccines. On December 2, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) became the first medicine regulator to approve an mRNA vaccine, authorizing the Pfizer-BioNTech vaccine for widespread use. [9] [10] [11] On December 11, the US Food and Drug Administration (FDA) issued an emergency use authorization for the Pfizer-BioNTech vaccine [12] [13] and one week later. similarly approved the Moderna vaccine.
The first successful transfection of mRNA packaged into a liposomal nanoparticle in a cell was published in 1989. [16] [17] A year later, "naked" (or unprotected) mRNA was injected into the muscle of mice. [3] [18] These studies were the first evidence that in vitro transcribed mRNA was capable of delivering the genetic information to produce proteins within living cell tissue [3] and led to the proposal of the concept of messenger RNA vaccines. [19] [20] [21]
In 1993, liposome-encapsulated mRNA was shown to stimulate T cells in mice. [22] [23] The following year, self-amplified mRNA was developed by including a viral antigen and a gene encoding replicase. [22] [24] The method was used in mice to elicit a humoral and cellular immune response against a viral pathogen. [22] The following year, mRNA encoding a tumor antigen was shown to elicit a similar immune response against cancer cells in mice. [25] [26]
Development
The first human clinical trial using ex vivo dendritic cells transfected with mRNA encoding tumor antigens (therapeutic cancer mRNA vaccine) began in 2001. [27] [28] Four years later, the successful use of modified nucleosides as a method of transporting mRNA within cells without activating the body's defense system. [27] [29] In 2008, the results of clinical trials of an mRNA vaccine injected directly into the body against cancer cells were reported. [30] [31]
BioNTech in 2008 and Moderna in 2010 were founded to develop mRNA biotechnologies. [32] [33] The US research agency DARPA at this time launched the ADEPT biotechnology research program to develop emerging technologies for the US military. [34] [35] The agency recognized the potential of nucleic acid technology for defense against pandemics and began investing in the field. [34] The DARPA grants were viewed as a vote of confidence which in turn encouraged other government agencies and private investors to invest in mRNA technology. [35] DARPA awarded a $ 25 million grant to Moderna at that time. [36]
The first human clinical trials using an mRNA vaccine against an infectious agent (rabies) began in 2013. [37] [38] During the following years, clinical trials of mRNA vaccines for several other viruses began. Human mRNA vaccines have been studied for infectious agents such as influenza, Zika virus, cytomegalovirus, and Chikungunya virus. [39] [40]
Acceleration
The COVID-19 pandemic and the sequencing of the causative virus SARS-CoV-2 in early 2020 led to the rapid development of the first approved mRNA vaccines. [41] [42] BioNTech and Moderna in December of the same year obtained approval for their mRNA-based COVID-19 vaccines. On December 2, seven days after its last eight-week trial, the UK's Medicines and Healthcare Products Regulatory Agency became the first global drug regulator in history to approve an mRNA vaccine, granting an authorization of Emergency for the BNT162b2 COVID-19 vaccine from Pfizer-BioNTech for widespread use. [43] [44] [45] On December 11, the FDA granted an emergency use authorization for the Pfizer - BioNTech COVID-19 vaccine and a week later a similar approval for the Modern COVID-19 vaccine.